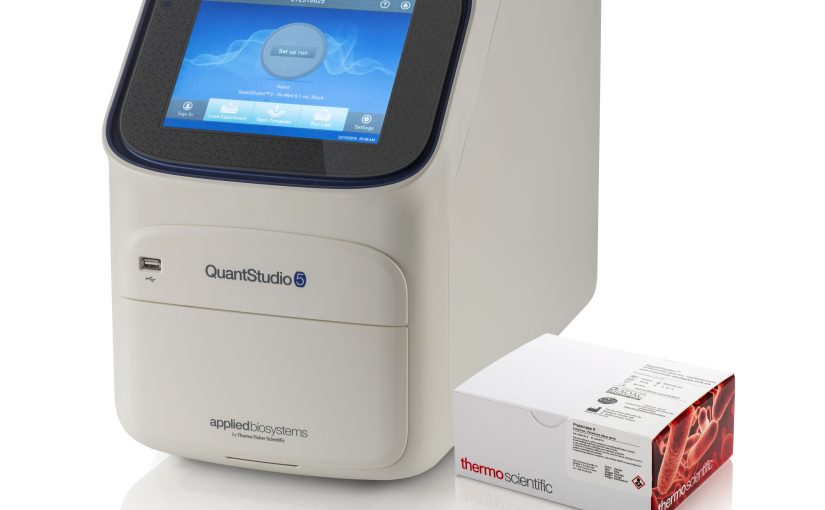
MicroVal has approved the issuing of the certificate for ThermoFisher Scientific’s SureTect Salmonella Species PCR Assay. Q Laboratories carried out the extension validation study for a broad range of foods in which the use of pre-warmed (41.5 or 37 ± 1 °C) BPW ISO as the primary enrichment media was evaluated, in addition to alternative sample sizes.
The Thermo Scientific SureTect Salmonella Species PCR Assay was originally validated by AFNOR according to the ISO 16140:2003 standard in 2013. Multiple extension studies were conducted since the initial validation study, and the first renewal validation enabled alignment with the revised ISO 16140 – 2:2016 standard in 2017. The method is validated for the use of three instruments: Applied Biosystems 7500 Fast, Applied Biosystems Quantstudio 5 and the PikoReal instrument. A summary of the initial validation, original renewal, and previous extension studies can be found on the available summary report (AFNOR Certificate: UNI 03/12-01/18).
All data generated from the three claimed thermocyclers for each matrix claimed under the AFNOR Certificate: UNI 03/12-01/18 was presented in a Gap Analysis report to the MicroVal Technical Committee (MVTC) according to the MicroVal Rules set up by the MicroVal General Committee (MGC). The MVTC agreed that the supporting data on the three different cyclers covered a broad range of foods, thus meeting the MicroVal requirements needed for the method to be transferred to become MicroVal 2022LR111. It should be noted that both Applied Biosystems 7500 Fast and QuantStudio 5 thermocyclers are included in the extension claims in opposite to the PikoReal instrument that was used in the ILS portion of the original validation.
The summary report of the extension validation study, available on the MicroVal website, provides an overview of the categories and sample sizes that were included in the final scope, as well as the instrumentation that was validated for use in this extension. The certificate and the accompanying summary report, number 2022LR111, can be found under ‘issued certificates / alternative methods’.